Boyle's Law
Robert Boyle is what the Boyle's Law is named after.
In 1655, Boyle moved to Oxford where he made Robert Hooke his assistant and together they constructed the most famous piece of experimental equipment related with Boyle, the vacuum chamber or air-pump.
This law appears in an appendix written in 1662 to his work New Experiments Physio-Mechanical, Touching the Spring of the Air and its Effects in 1660 (Hunter).
This law appears in an appendix written in 1662 to his work New Experiments Physio-Mechanical, Touching the Spring of the Air and its Effects in 1660 (Hunter).
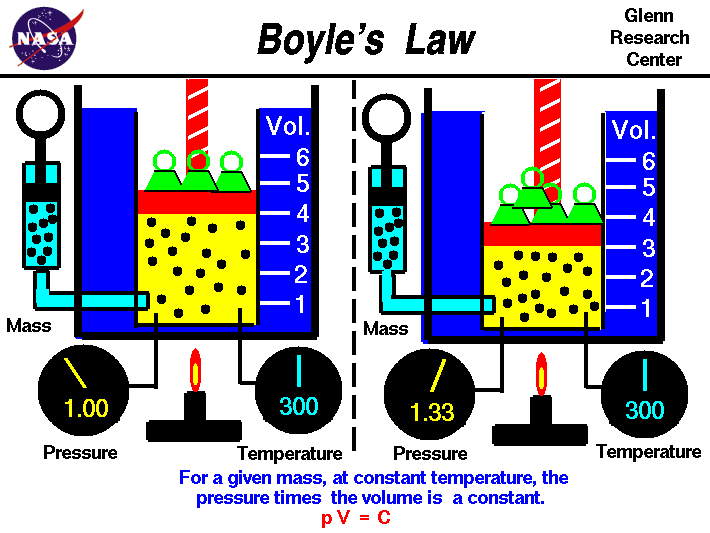
This law describes the relationship between pressure and volume of gases, so if the pressure is doubled then the volume will be halved.
Some examples of the relationship between pressure and volume are:
-The bubbles exhaled by a scuba diver grow as the approach the surface of the ocean. (The pressure exerted by the weight of the water decreases with depth, so the volume of the bubbles increases as they rise.)
-Deep sea fish die when brought to the surface. (The pressure decreases as the fish are brought to the surface, so the volume of gases in their bodies increases, and pops bladders, cells, and membranes).


Some examples of the relationship between pressure and volume are:
-The bubbles exhaled by a scuba diver grow as the approach the surface of the ocean. (The pressure exerted by the weight of the water decreases with depth, so the volume of the bubbles increases as they rise.)
-Deep sea fish die when brought to the surface. (The pressure decreases as the fish are brought to the surface, so the volume of gases in their bodies increases, and pops bladders, cells, and membranes).
This is a graph of the relationship between volume and pressure.
This law determines that for the same amount of a gas at constant temperature, P*V=constant.
P = pressure (measured in atm)
V = volume (measured in Liters)
This equation is derived from the Ideal Gas Law equation P * V = n * R * T
^ ^ ^
pressure x volume = constant
Example problem:
A gas has a pressure of 1.01 atm and occupies a volume of 6.4 L. If the gas is compressed to a volume of 1.05 L, what will its pressure be, assuming constant temperature? Answer in units of atm.
Explanation:
V1 = 6.4 L V1 = 1.05 L
V = volume (measured in Liters)
This equation is derived from the Ideal Gas Law equation P * V = n * R * T
^ ^ ^
pressure x volume = constant
Example problem:
A gas has a pressure of 1.01 atm and occupies a volume of 6.4 L. If the gas is compressed to a volume of 1.05 L, what will its pressure be, assuming constant temperature? Answer in units of atm.
Explanation:
V1 = 6.4 L V1 = 1.05 L
P2 = 1.01 atm P2 = ?
Applying Boyle's Law,
P1V1 = P2V2
P1 V1 (1.01 atm) (6.4 L)
P2 = ------ = --------------------
V2 1.05 L
= 6.15619 atm
Sample problems:
1. The initial pressure was 3 atm while the volume was 2 L. What would the volume be if the pressure rose to 6 atm?
2. The initial pressure was 380 atm while the volume was 6 L. What would the volume be if the pressure rose to 760 atm?
3. The initial pressure was 5.2 atm while the volume was 0.555 L. What would the pressure be if the volume rose to 2.14 atm?
4. The initial volume and pressure of the gas is 2L, 3 atm. Assuming the temperature and moles of gas is constant, what is the pressure if the volume is reduced to 1.25 L?
5. Four liters of carbon dioxide have a pressure of 1.5 atmospheres. If the original pressure was .9 atmospheres, what was the original volume?
Answer Key:
1. V2 = 1L
2. V2 = 3L
3. P2 = 1.349 atm
4. P2 = 4.8 atm
5. V₁= 6.666... L
sources:
campus.udayton.edu/~hume/Boyle/boyle.htm
http://antoine.frostburg.edu/chem/senese/101/gases/faq/everyday-gas-laws.shtml
graph: www.citycollegiate.com
Created by: Celesta Monsivaiz
http://antoine.frostburg.edu/chem/senese/101/gases/faq/everyday-gas-laws.shtml
graph: www.citycollegiate.com
Created by: Celesta Monsivaiz
No comments:
Post a Comment