Chemical Reactions
Goes over:
1. Balancing chemical equations
2. Writing skeleton equations
3. Redox reaction
4. Classify reactions
5. Predict the products
Balancing chemical equations
Law conservation of mass states that matter can be changed from one form into another, mixtures can be separated or made, and pure substances can be decomposed, but the total amount of mass remains constant.
To balance equations there has to be the same number of atoms for each element on both sides of the equation.
1) _SiI4 + _ Mg → _ Si + _MgI2
This is not a balanced equation because if you write down the atoms for each element on both sides of the equation it will be unequal:
Reactants Products
Si _1_ Si _1_
I _4_ I _2_
Mg _1_ Mg _1_
Iodine doesn't have the same number of atoms, so to balance this equation place coefficients in front of the terms to change the number of atoms.
1) _SiI4 + _2_Mg → _ Si + _2_MgI2
To see if it's balance write down the number of atoms for each element on both sides of the equation:
Reactants. Products
Si _1_. Si _1_
I _4_. I _4_ (the coefficient 2 is multiplied with I2 to produce 4)
Reactants Products
Si _1_ Si _1_
I _4_ I _2_
Mg _1_ Mg _1_
Iodine doesn't have the same number of atoms, so to balance this equation place coefficients in front of the terms to change the number of atoms.
1) _SiI4 + _2_Mg → _ Si + _2_MgI2
Reactants. Products
Si _1_. Si _1_
I _4_. I _4_ (the coefficient 2 is multiplied with I2 to produce 4)
Mg _2_ M_2_ (the coefficient 2 multiplies with an invisible 1 to get 2)
Now it is balanced!
2) _ (NH4)2O + _Cr(BrO3)3 → _Cr2O3 + _ NH4BrO3
Let's see if it's balanced:
Reactants Products
N_2_ N_1_
H_8_ H_4_
O_10_ O_6_
Cr_1_ Cr_2_
Br_3_ Br_1_
*Now when there are parenthesis the subscript is distributed to the elements inside the parenthesis.
Nitrogen, Hydrogen, Oxygen, Chromium, and Bromine are not balanced, let's balance them.
2) _3_ (NH4)2O + _2_Cr(BrO3)3 → _Cr2O3 + _6_ NH4BrO3
More examples of unbalanced equations being balanced using coefficients
3) _K3PO4 + _HCl → _KCl + _H3PO4
_K3PO4 + _3_HCl → _3_KCl + _H3PO4
4) _C3H6 + _ O2 → _CO2 + _H2O
_2_C3H6 + _9_ O2 → _6_CO2 + _6_H2O
5) _Ca(ClO3)2 → _CaCl2 + _O2
_Ca(ClO3)2 → _CaCl2 + _3_O2
1) potassium iodide+ lead (II) nitrate → potassium nitrate + lead (II) iodide
KI + Pb(NO3)2 → KNO3 + PbI2
Balance it:
_2_KI + _Pb(NO3)2 → _2_KNO3 + PbI2
2) mercury (II) oxide → mercury + oxygen
HgO → Hg + O
It's already balanced because each element has the same number of atoms on both sides.
3) calcium + aluminum chloride → calcium chloride + aluminum
Ca + AlCl3 → CaCl2 + Al
Balance it:
_3_Ca + _2_AlCl3 → _3_CaCl2 + _2_Al
4) mercury (I) nitrate + sodium carbonate → sodium nitrate + mercury (I) carbonate
HgNO3 + Na4C → NaNO3 + Hg4C
Balance it:
_4_HgNO3 + _Na4C → _4_NaNO3 + Hg4C
5) potassium bromide + aluminum nitrate → potassium nitrate + aluminum bromide
KBr + Al(NO3)3 → KNO3 + AlBr3
Balance it:
_3_KBr + Al(NO3)3 → _3_KNO3 + AlBr3
Sources
http://www.chem.wisc.edu/deptfiles/genchem/sstutorial/Text1/Tx14/tx14
http://chemistry.tutorvista.com/inorganic-chemistry/redox-potential.html?view=simple
http://chemistry-group4.blogspot.com/2010/03/decomposition
http://citadel.sjfc.edu/students/jas05321/e-port/Reactions%20Study%20Guide.html
Now it is balanced!
2) _ (NH4)2O + _Cr(BrO3)3 → _Cr2O3 + _ NH4BrO3
Let's see if it's balanced:
Reactants Products
N_2_ N_1_
H_8_ H_4_
O_10_ O_6_
Cr_1_ Cr_2_
Br_3_ Br_1_
*Now when there are parenthesis the subscript is distributed to the elements inside the parenthesis.
Nitrogen, Hydrogen, Oxygen, Chromium, and Bromine are not balanced, let's balance them.
2) _3_ (NH4)2O + _2_Cr(BrO3)3 → _Cr2O3 + _6_ NH4BrO3
More examples of unbalanced equations being balanced using coefficients
3) _K3PO4 + _HCl → _KCl + _H3PO4
_K3PO4 + _3_HCl → _3_KCl + _H3PO4
4) _C3H6 + _ O2 → _CO2 + _H2O
_2_C3H6 + _9_ O2 → _6_CO2 + _6_H2O
5) _Ca(ClO3)2 → _CaCl2 + _O2
_Ca(ClO3)2 → _CaCl2 + _3_O2
Writing skeleton equations
To write a skeleton equation just write the symbols that represent the names of the substance and put subscripts that are necessary. You don't have to balance skeleton equations unless asked to do so.1) potassium iodide
KI + Pb(NO3)2 → KNO3 + PbI2
Balance it:
_2_KI + _Pb(NO3)2 → _2_KNO3 + PbI2
2) mercury (II) oxide → mercury + oxygen
HgO → Hg + O
It's already balanced because each element has the same number of atoms on both sides.
3) calcium + aluminum chloride → calcium chloride + aluminum
Ca + AlCl3 → CaCl2 + Al
Balance it:
_3_Ca + _2_AlCl3 → _3_CaCl2 + _2_Al
4) mercury (I) nitrate + sodium carbonate → sodium nitrate + mercury (I) carbonate
HgNO3 + Na4C → NaNO3 + Hg4C
Balance it:
_4_HgNO3 + _Na4C → _4_NaNO3 + Hg4C
5) potassium bromide + aluminum nitrate → potassium nitrate + aluminum bromide
KBr + Al(NO3)3 → KNO3 + AlBr3
Balance it:
_3_KBr + Al(NO3)3 → _3_KNO3 + AlBr3
Redox reaction
A redox reaction (oxidation-reduction reactions) is a reaction in which electrons are transferred. To identify what has been oxidized and reduced in a redox reaction look for a change in the oxidation number of an element. In oxidation the oxidation number increases. In reduction the oxidation number decreases.
How to find the oxidation number of the bold element:
1) I2 Since it is an element by itself it has an oxidation of 0.
2) Cr2O7^-2 There is always an element to start with when trying to figure out the oxidation number. This is the order from which you start with oxygen, hydrogen, group 1A, 2A, Ag, Zn, Cd, Al, and group 7A.
In this equation start with oxygen. Oxygen has a oxidation number of -2 then multiply it with its subscript 7. Then equal this to -2 (its charge), the missing number to make this equation equal -2 is -12. Divide -12 with 2 (the subscript of Cr) to give you -6, the oxidation number of Cr.
3) HMnO4 Oxygen has an oxidation number of -2, multiply it with its subscript 4. Since the middle element is the unknown oxidation number, take the oxidation number of the left element (hydrogen has oxidation number of +1) and add it to the equation. The equal is to 0 (if it doesn't have a charge it is always equal to 0), the missing number is -7, the oxidation number of Mn.
1) 2 Rb + 3 F2 --> 2 RbF
oxidation: 0 0 +1 -1
This is a redox reaction because there was a change of oxidation. Since Rb oxidation number increased it is oxidized. Since F decreased it's reduced.
2) 2 K + 1 Zn(NO3)2 --> 1 Zn + 2 KNO3
oxidation: 0 -8 6 -2 0 +1 +5 -2
This is a redox reaction because there was a change of oxidation. Since Zn and K oxidation number increased it's oxidized. Since N decreased it's reduced.
3) 1 CaCO3 --> 1 CaO + 1 CO2
oxidation: +2 +4 -2 +2 -2 +4 -2
This is not redox because all oxidation numbers stayed the same.
Classify reactions
Synthesis: two elements as reactants to make one product. Think of it like eating a sandwich then drinking a smoothie and it mixes together in your stomach in the end.
Decomposition: one reactant breaks apart into its elements. Think of it like a peanut, you break it apart to get to separate things.
Single replacement: characterized by having an element and a compound as reactants and the products are another element and another compound. Think of it as a giraffe, the one with the longer neck takes the place to breed then with one with a shorter neck.
Combustion: a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Think of it like dough and cows to make cookies and milk (no avoiding it).
1) I2 Since it is an element by itself it has an oxidation of 0.
2) Cr2O7^-2 There is always an element to start with when trying to figure out the oxidation number. This is the order from which you start with oxygen, hydrogen, group 1A, 2A, Ag, Zn, Cd, Al, and group 7A.
In this equation start with oxygen. Oxygen has a oxidation number of -2 then multiply it with its subscript 7. Then equal this to -2 (its charge), the missing number to make this equation equal -2 is -12. Divide -12 with 2 (the subscript of Cr) to give you -6, the oxidation number of Cr.
3) HMnO4 Oxygen has an oxidation number of -2, multiply it with its subscript 4. Since the middle element is the unknown oxidation number, take the oxidation number of the left element (hydrogen has oxidation number of +1) and add it to the equation. The equal is to 0 (if it doesn't have a charge it is always equal to 0), the missing number is -7, the oxidation number of Mn.
1) 2 Rb + 3 F2 --> 2 RbF
oxidation: 0 0 +1 -1
This is a redox reaction because there was a change of oxidation. Since Rb oxidation number increased it is oxidized. Since F decreased it's reduced.
2) 2 K + 1 Zn(NO3)2 --> 1 Zn + 2 KNO3
oxidation: 0 -8 6 -2 0 +1 +5 -2
This is a redox reaction because there was a change of oxidation. Since Zn and K oxidation number increased it's oxidized. Since N decreased it's reduced.
3) 1 CaCO3 --> 1 CaO + 1 CO2
oxidation: +2 +4 -2 +2 -2 +4 -2
This is not redox because all oxidation numbers stayed the same.
Classify reactions
Synthesis: two elements as reactants to make one product. Think of it like eating a sandwich then drinking a smoothie and it mixes together in your stomach in the end.
2Ca + O2 --> 2CaO
Decomposition: one reactant breaks apart into its elements. Think of it like a peanut, you break it apart to get to separate things.
2H2O2 --> 2H2O + O2
Single replacement: characterized by having an element and a compound as reactants and the products are another element and another compound. Think of it as a giraffe, the one with the longer neck takes the place to breed then with one with a shorter neck.
Fe + CuSO4 --> Cu + FeSO4
Combustion: a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Think of it like dough and cows to make cookies and milk (no avoiding it).
CH4 + 2O2 --> CO2 + 2H2O
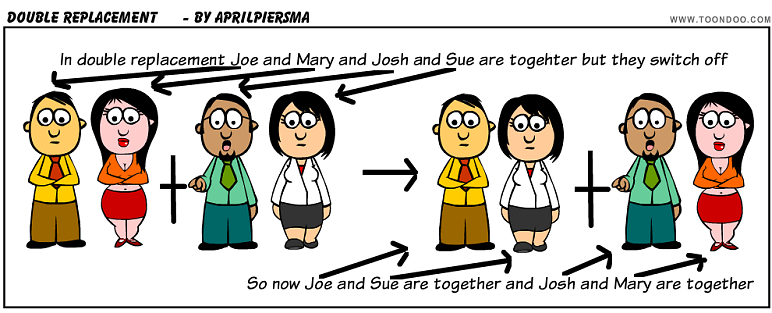
Double replacement: two compounds as reactants and the products are two other compounds. Think of it like its Halloween and you and your friend switch candy.
1)AlCl3(aq) + Na2SO4(aq) --> Al2(SO4)3(s) + NaCl(aq)
When balanced:
_2_AlCl3(aq) + _3_Na2SO4(aq) --> Al2(SO4)3(s) + _6_NaCl(aq)
Since Cl was with Al then ended up with Na and SO4 was with Na then ended up with Al it is a double replacement.
2) Zn(s) + S8(s) --> ZnS(s)
When balanced: _8_Zn(s) + S8(s) --> _8_ZnS(s)
Since the reactants Zn and S come together to form one compound as a product it is showing synthesis.
3) Al2S3(s) --> Al(s) + S8(s)
When balanced: _8_Al2S3(s) --> _16_Al(s) + _3_S8(s)
Since the compound AlS separated to produce Al and S it showed decomposition.
4) H2SO4(aq) + Fe(s) --> H2(g) + FeSO4(aq)
It's already balanced, atoms of each element on either side of the equation has the same number of atoms.
In the equation Fe takes the place of H creating FeSO4 because it is more active, this shows single replacement.
5) C12H22O11(g) + O2(g) --> CO2(g) + H2O(g)
When balanced: C12H22O11(g) + _12_O2(g) --> _12_CO2(g) + _11_H2O(g)
In the equation the products are CO2 and H2O. When the reactants contain C, H and O and the products are carbon dioxide and water, combustion has occurred.
NaCl + KBr --> NaBr + KCl
1)AlCl3(aq) + Na2SO4(aq) --> Al2(SO4)3(s) + NaCl(aq)
When balanced:
_2_AlCl3(aq) + _3_Na2SO4(aq) --> Al2(SO4)3(s) + _6_NaCl(aq)
Since Cl was with Al then ended up with Na and SO4 was with Na then ended up with Al it is a double replacement.
2) Zn(s) + S8(s) --> ZnS(s)
When balanced: _8_Zn(s) + S8(s) --> _8_ZnS(s)
Since the reactants Zn and S come together to form one compound as a product it is showing synthesis.
3) Al2S3(s) --> Al(s) + S8(s)
When balanced: _8_Al2S3(s) --> _16_Al(s) + _3_S8(s)
Since the compound AlS separated to produce Al and S it showed decomposition.
4) H2SO4(aq) + Fe(s) --> H2(g) + FeSO4(aq)
It's already balanced, atoms of each element on either side of the equation has the same number of atoms.
In the equation Fe takes the place of H creating FeSO4 because it is more active, this shows single replacement.
5) C12H22O11(g) + O2(g) --> CO2(g) + H2O(g)
When balanced: C12H22O11(g) + _12_O2(g) --> _12_CO2(g) + _11_H2O(g)
In the equation the products are CO2 and H2O. When the reactants contain C, H and O and the products are carbon dioxide and water, combustion has occurred.
Predict the products
A net ionic equation includes only the components of the reaction that undergo a change.
To write a net ionic equation:
1) write a molecular equation (you do not need to balance it yet)
2) break apart into ions all strong acids, strong bases, and soluble salts (unless they are present as a solid, liquid, or gas).
3) make sure to leave all solids, liquids, and gases together
4) Remove all spectator ions (= ions that occur unchanged in any way from the reactant side to the product side)
To write a net ionic equation:
1) write a molecular equation (you do not need to balance it yet)
2) break apart into ions all strong acids, strong bases, and soluble salts (unless they are present as a solid, liquid, or gas).
3) make sure to leave all solids, liquids, and gases together
4) Remove all spectator ions (= ions that occur unchanged in any way from the reactant side to the product side)
Sources
http://www.chem.wisc.edu/deptfiles/genchem/sstutorial/Text1/Tx14/tx14
http://chemistry.tutorvista.com/inorganic-chemistry/redox-potential.html?view=simple
http://chemistry-group4.blogspot.com/2010/03/decomposition
http://citadel.sjfc.edu/students/jas05321/e-port/Reactions%20Study%20Guide.html